Research activities of the Tissue Electronics laboratory can be divided into three broad research areas, aiming mainly to use electrical active materials for stimulating cells for biomedical applications:
- Electrophysiology of 3D cellular architectures
- Photovoltaic biomaterials development for skin regeneration
- Engineering Bioelectronics interfaces
Electrophysiology of 3D cellular architectures
3D devices (multi electrode arrays, CMOS)
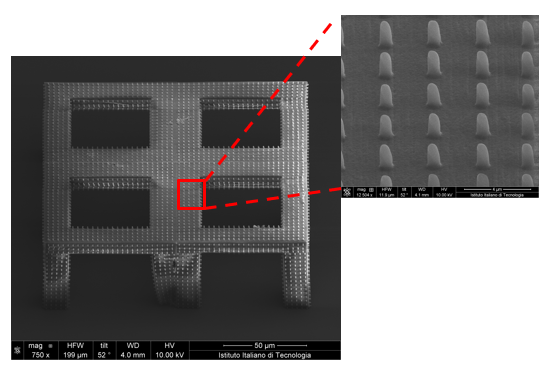

Electrophysiology has been used to study cell cultures in vitro for more than 40 years, revealing crucial aspects of the biology of cardiac and neuronal cells.
Following the current trend in Biomedical and Tissue Engineering, we seek to move from 2D cell cultures to a more realistic 3D environment which recapitulates more of the natural cells’ milieu. Our goal is to conduct electrophysiological experiments on three-dimensional cultures. We are currently approaching this aim with two different approaches: by fabricating 3D electrodes and by developing 3D culture scaffolds for electrogenic cells.
Photovoltaic biomaterials development for skin regeneration
3D biocompatible and flexible photovoltaic nanopillar-based
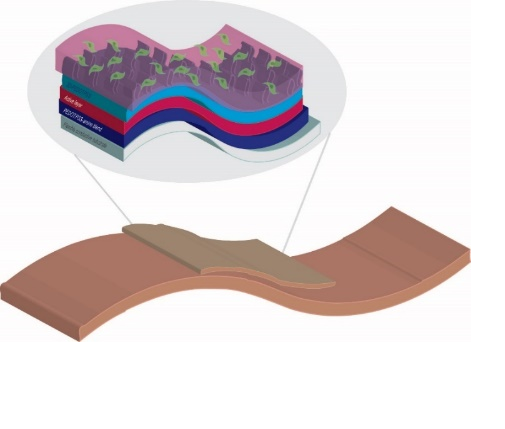
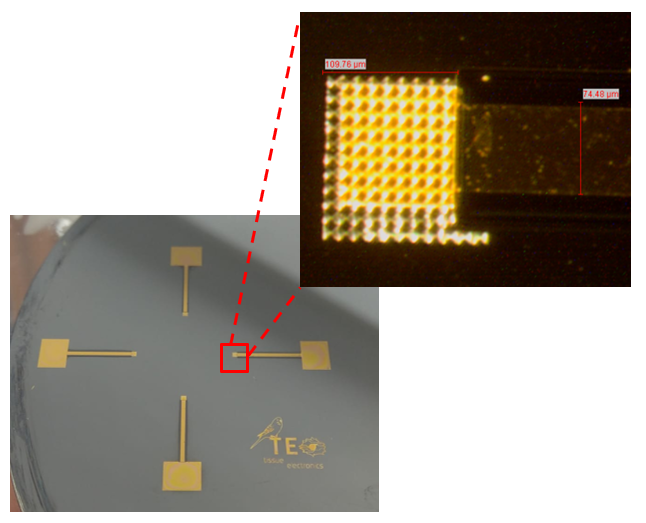
Light and electrical stimulation in regenerative medicine is a well-known approach, especially for skin and nerve regeneration. Among all the techniques employed to induce a cell photostimulation, the use of the photocurrent produced by a photovoltaic device, capable to interact directly with cells, represent one of the more promising, challenging and minimally invasive approaches.
Our research aims at the development of innovative materials able to work as biointerface between cells (fibroblasts) and a photovoltaic device. The perfect candidate to carry out this task can be a conductive polymer due to their feasibility of applying electrical stimuli. In the light of above, an appropriate modulation of the composition of PEDOT/PSS conductive polymers, by adding additives to improve conductivity (EG and PEG), functionalizing it with biological molecules (SLBs) or/and changing the morphology of the surface, can be the good way to obtain an high performing substrate which could combine the optoelectronic properties with tissue compatibility.
Both for the synthesis and the characterization very advanced tools and techniques are used such as Reactive-ion etching (RIE), the Focused-Ion-Beam Scanning Electron Microscopy (SEM-FIB)We are actively addressing the following objectives:
- Synthesis and characterization of a biocompatible and flexible 3D based on photovoltaic nanoparticles
- Synthesis of PEDOT / PSS functionalized with SLBs
- Building of a flexible and biocompatible photovoltaic device
Engineering Bioelectronics interfaces
3D cellular architectures (scaffold-based) of neuronal and cardiac cells
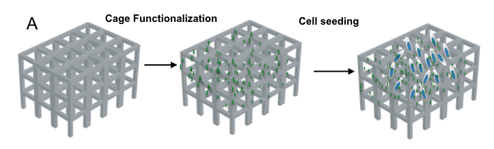
Regeneration and production of functionally viable tissue is the ultimate goal in tissue engineering. Cell fate is largely determined by interactions that occur at the interface between cells and their surrounding microenvironment. Conventional cell culture provides unnatural conditions with only 2D space for growing cells that leads to the development of physiologically and topologically compromised cells. Tissue/cells in the body on the contrary, grow in three dimensions surrounded by extra-cellular matrix (ECM) and other cells bathed in blood plasma or tissue fluid. Culturing cells ex vivo that differentiate and maintain in vivo characteristics is important not only for the deep knowledge of cell function but also in tissue engineering and regenerative medicine. For this reasons we have the needed to develop 3D system/scaffold that better replicate the structural organization of living tissue and understand in which way they interact with biomaterials. Using the electron microscopy (SEM/FIB) in combination with fluorescence-based techniques we are able to achieve the highest resolution to monitor the cell behavior of electrogenic cells (neuronal and cardiac cells) to pursue also an electrophysiological approach.
Neuromorphic
Neuromorphic devices represent a powerful platform to recreate artificial neural networks, which will offer the chance to improve implantable devices towards the replacement and repair of injured brain areas. Our innovative approach consists in reproducing an electrochemical synapse coupling dopaminergic cells with conductive organic transistors (realized with PEDOT:PSS as sensitive layer) in order to realize a two-way system: infact, these devices have a clear “learning” process from the outer stimuli, changing their state and giving a different response similarly to physiological synaptic plasticity. The coupling of several analysis techniques (fluorescence microscopy, SEM/FIB and electrical characterization) offer us the chance to have a very detailed profile of the cell-material interface and of the response of our device:
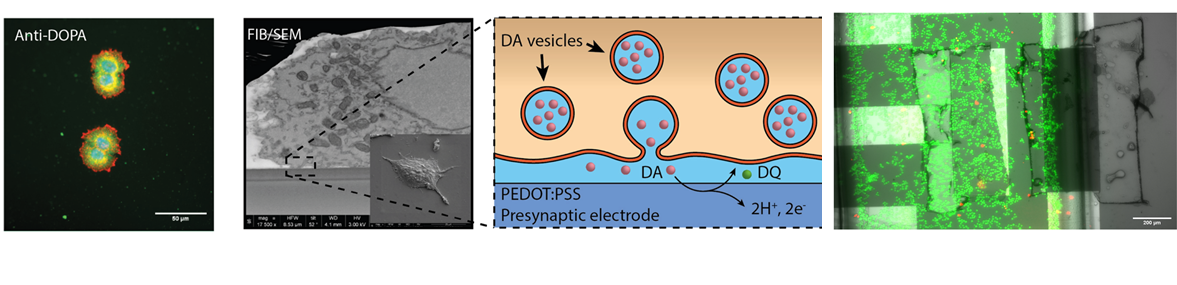
Advanced microscopy for interface investigation
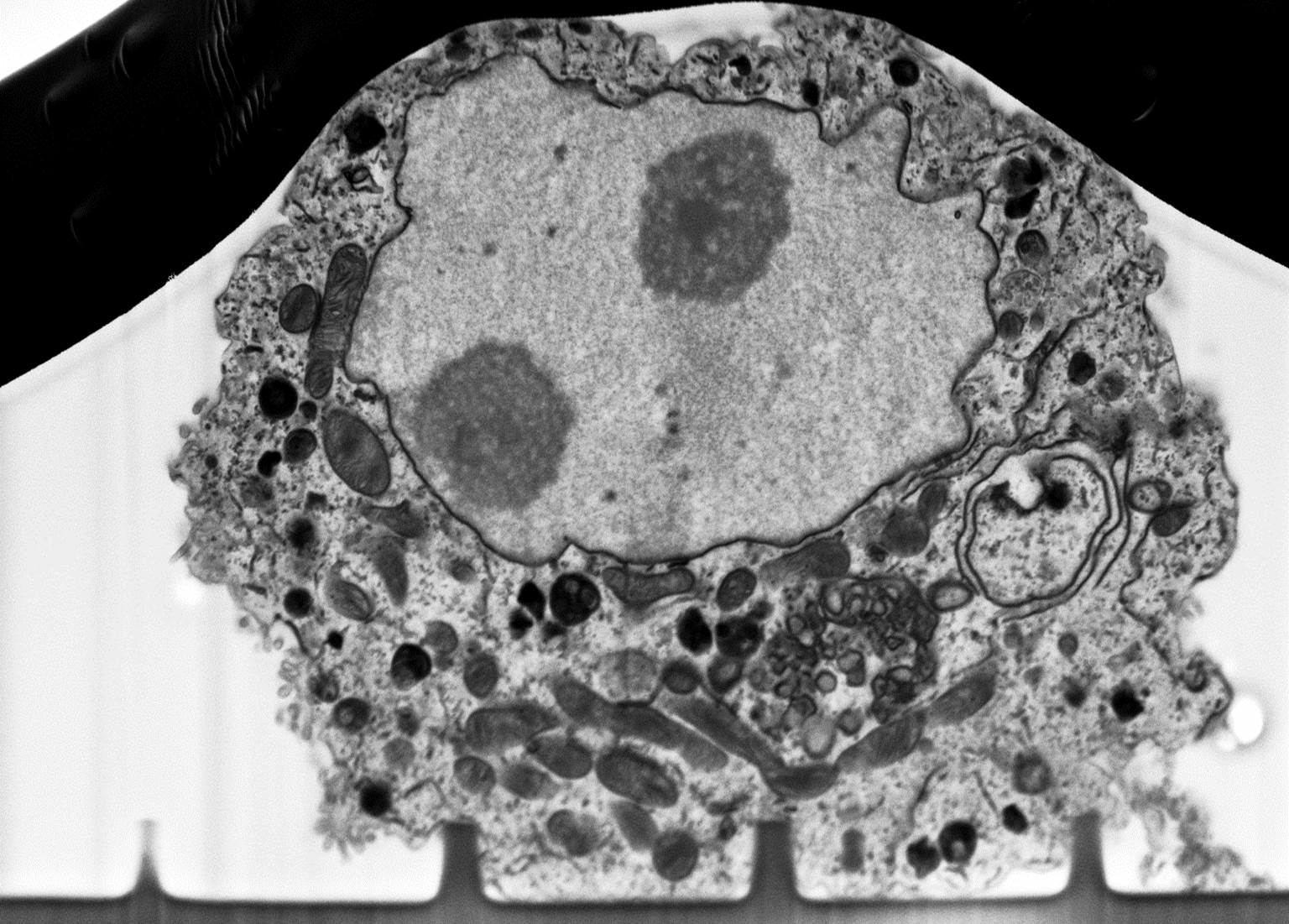
The interplay that takes place between cells and scaffold/biosensor is crucial in terms of biocompatibility or efficacy of the device. From one side, cell instructive scaffolds are designed as guidance for cell with specific topographies, from the other side cell-biosensor coupling strongly depends on how close the cells are onto its surface. In both scenarios, cell membrane is the main active protagonist by means of its bending. Therefore, advanced microscopy helps to clearly resolve the membrane-to-material interface with high resolution at a nanometre length scale (~ 5 nm). First the cell/biosensor or cell/scaffold system is prepared with ultrathin plasticization procedure (UTP). Afterwards, cell-material cross section is exposed by milling the abovementioned system with a focused ion beam (FIB), and then by scanning its surface with an electron beam (SEM). UTP and SEM/FIB coupling method allows to:
- identify directly a region of interest (ROI) to cut
- bypass the substrate removal, sometimes required for transmission electron microscopy samples
- preserve subcellular structures due to plastic layer embedding
- establish the cleft between cells and scaffold/biosensor
- have an inside look to cellular ultrastructures
- perform the 3D intracellular space reconstruction.
Prova
